Scientists starved worms — then discovered the switch that controls aging
Powerful model to study senescence.
- Date:
- July 4, 2025
- Source:
- Max Planck Institute for Biology of Ageing
- Summary:
- Scientists have discovered that starving and then refeeding worms can reveal surprising secrets about aging. When a specific gene (called TFEB) is missing, these worms don’t bounce back from fasting—they instead enter a state that looks a lot like aging in humans, with signs of stress and cell damage. This research gives scientists a simple but powerful way to study how aging begins—and how it might be stopped. Even more intriguing, the same process might help explain how some cancer cells survive treatment by going into a kind of sleep mode.
- Share:
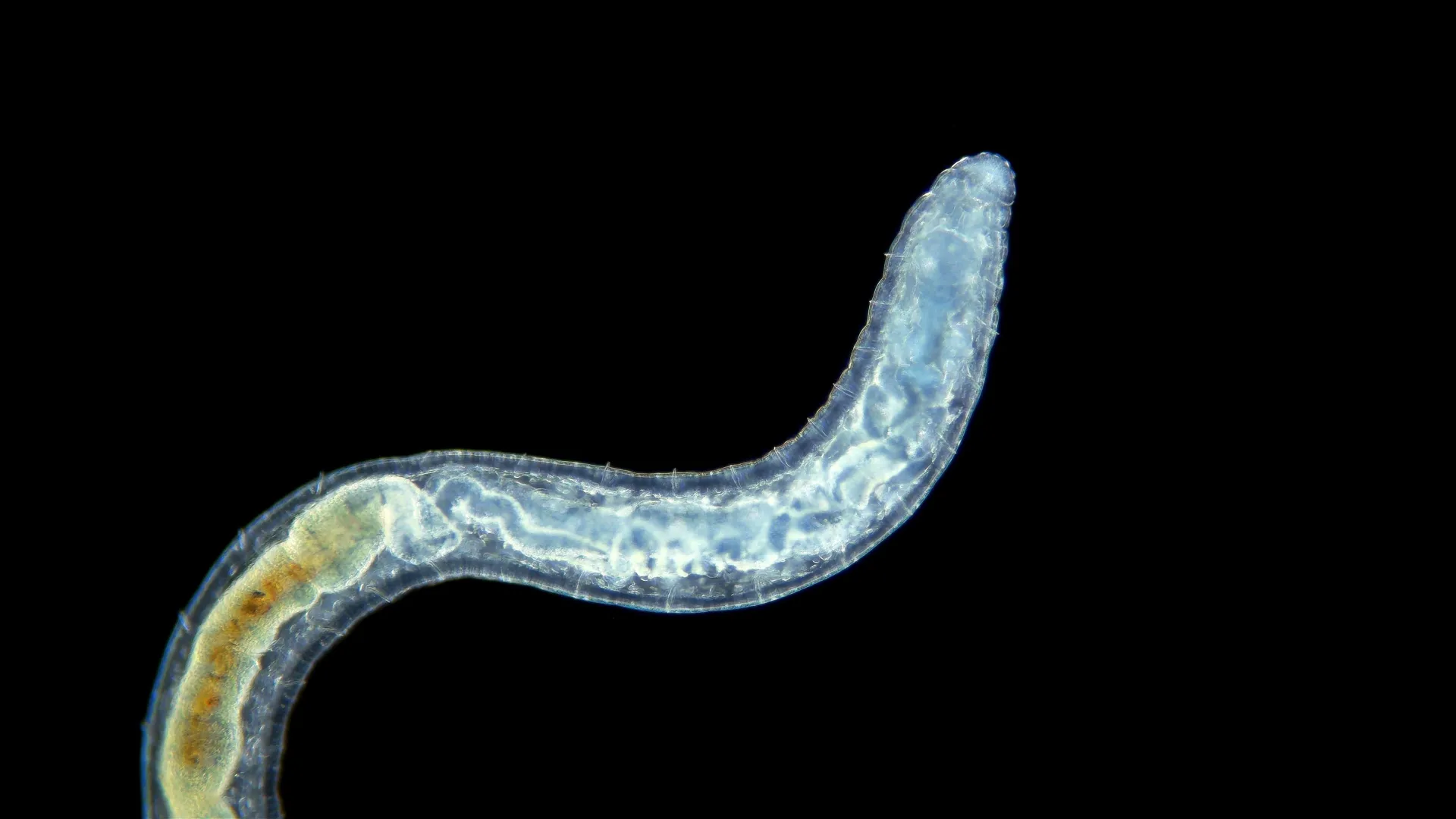
The researchers induced the senescent-like state in worms by manipulating the transcription factor TFEB. Under normal conditions, worms subjected to long-term fasting followed by refeeding regenerate and appear rejuvenated. However, in the absence of TFEB, the worm's stem cells fail to recover from the fasting period and instead enter a senescent-like state. This state is characterised by markers such as DNA damage, nucleolus expansion, mitochondrial reactive oxygen species (ROS), and the expression of inflammatory markers, which are similar to those observed in mammalian senescence.
"We present a model for studying senescence at the level of the entire organism. It provides a tool to explore how senescence can be triggered and overcome," explains Adam Antebi, head of the study and director at the Max Planck Institute for Biology of Ageing.
The TFEB-growth factor axis
TFEB is a transcription factor involved in cellular responses to nutrient availability. It plays a crucial role in responding to fasting by regulating gene expression. In its absence, worms attempt to initiate growth programs without sufficient nutrients, leading to senescence.
"With our new model, we conducted genetic screens to identify mutations that can circumvent senescence. We identified growth factors, including insulin and transforming growth factor beta (TGFbeta), as the key signaling molecules that are dysregulated upon TFEB loss," Antebi explains.
The TFEB-TGFbeta signaling axis is also regulated during cancer diapause, a state in which cancer cells remain in a dormant, non-dividing condition to survive chemotherapy. In the future, the researchers want to test whether their worm model can be used to find new treatments targeting senescent cells during aging as well as cancer dormancy.
Story Source:
Materials provided by Max Planck Institute for Biology of Ageing. Note: Content may be edited for style and length.
Journal Reference:
- Tim J. Nonninger, Jennifer Mak, Birgit Gerisch, Valentina Ramponi, Kazuto Kawamura, Roberto Ripa, Klara Schilling, Christian Latza, Jonathan Kölschbach, Manuel Serrano, Adam Antebi. A TFEB–TGFβ axis systemically regulates diapause, stem cell resilience and protects against a senescence-like state. Nature Aging, 2025; DOI: 10.1038/s43587-025-00911-4
Cite This Page: